



The mitral valve is critical for maintaining optimal directional blood flow within the heart. A malfunction, known as mitral regurgitation, allows blood to flow backward, severely affecting about 10% of the global population over 75 years old. Currently, the only effective solution is open-heart surgery, yet this is feasible for only 20% of those affected. Without proper intervention, this condition proves fatal for half of the patients within four years. In response to this reality, non-surgical valve replacement alternatives have been developed to meet this critical need.
However, the rollout of these pioneering solutions is hindered by significant technological obstacles, especially the complexity of cardiac anatomy. Adapting non-surgical valvular implant devices to the variety of anatomical configurations remains a challenge, thus limiting to 15% the number of patients who can benefit from these innovations. This highlights the critical importance of developing innovative technologies designed to accommodate a broad range of patients, thereby significantly expanding access to care.
This sector represents a significant opportunity, by 2032, it is projected that approximately 8 million patients in the United States, Europe, and China will be able to benefit from these treatments, with the market for non-surgical mitral valve replacement estimated at $18 billion. Moreover, there is a strong demand from leading medtech companies for viable solutions.
Despite the high potential of the mitral and tricuspide valve replacement market, it currently lacks a device that is ready for commercialization.
This gap not only invites us to enter the market but to revolutionize it with our cutting-edge technology.
Previous attempts with the first generation have shown a low rate of patient eligibility to benefit from this technology is not an anomaly but a sign of incompatibility with the current and future needs of the market.
Our approach is not to follow but to lead with innovation. We aim to surpass first-generation technologies by tackling the critical barriers that have limited their success.
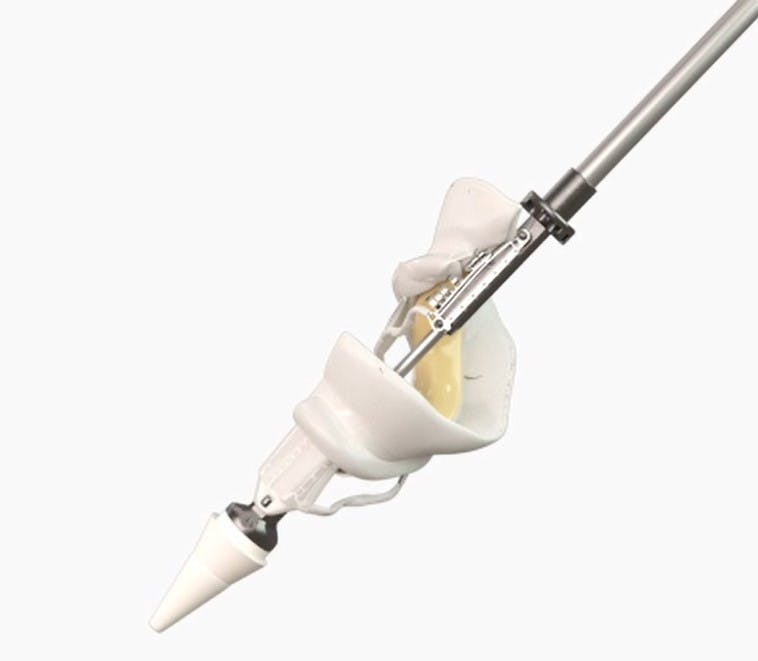
Our technology, which includes advanced ribbon valve technology and a dual frame design, promises to deliver valves of varying sizes through a minimally invasive, low-profile catheter. It is designed to accommodate the diverse anatomical configurations of the mitral and tricuspid annulus, prevent interference with cardiac outflow and obstruction, and ensure durability against cardiac motion while maintaining stable anchoring and effective sealing.
The Cornelis Valve from Open Stent Solution is transforming valvular treatment with its revolutionary design. Thanks to a unique open structure, it enables direct helical insertion, eliminating the need for compression and allowing the use of a thin catheter for valves of all sizes, including those exceeding 60 mm in diameter. This method simplifies implantation, minimizes risks, and ensures perfect adaptation to various anatomies.
With its patented and unique design that incorporates various technological innovations into one solution, the Cornelis Valve maximizes performance and safety, broadening treatment access for many patients and aiming for a dominant market position.

Launched in 2018, Open Stent Solution is driven by a seasoned, passionate team ready to tackle the challenges associated with mitral and tricuspid insufficiency. With expertise spanning all critical areas of success, this team of 11 professionals combines skills in engineering, research, technology, and medicine. Motivated by the desire to spark a medical revolution, they work together to develop groundbreaking innovations aimed at transforming patients' lives.
Our bench tests and animal model trials have confirmed the excellent hemodynamic performance of the valve, as well as the implant's optimal integration into the anatomical environment. These results are perfectly in line with the quality standards required by current regulations, demonstrating the effectiveness and reliability of our solution.
Our patent portfolio is the foundation of our innovation, protecting our unique technological progress in the medical sector. Made up of 15 patents divided into 4 distinct families, it provides extensive protection over a global territory. A freedom-to-operate analysis conducted by a renowned Californian firm has found no commercialization restrictions for our device, thereby reinforcing our ability to market our innovative solutions globally without infringement concerns.

Now is A timely opportunity for significant impact investment before the design is finalized. The public investment bank has greatly supported us with a deep tech grant of one million euros. As winners of the prestigious I-Nov competition, we received support of 1.6 million euros. This public funding adds to our pre-Series A financing round, where risk has already been significantly reduced. Goldenest Capital, the investment bank, anticipates that an investment at this stage could yield a return on investment twenty times higher (ROI X20).

This new concept consolidates all the technical solutions needed to overcome the hurdles that major players have to date not successfully navigated into a single design. The device from Open Stent Solution represents a real hope for numerous patients worldwide.
To develop its patented innovation, a seed funding of 2.5 million euros was secured from the French network Angels Santé and the Belgian BeAngels, as well as from private investors, including surgeons.
BPI France supported the project with a contribution of 1.5 million euros. These initial funds enabled us to finalize the development of our innovative system.
These advancements have brought the project to a level of maturity that further secures the risk/investment ratio.

France will be Europe's top destination for foreign investment in 2022 (EY, 2023)
The income tax reduction rate is set at 30% for subscriptions made between January 1, 2024, and December 31, 2028.
To invest click here: https://capitalcell.es/en/campaign/open-stent-solution/